The Boiling Point of a Given Liquid Varies
A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. What affect the boiling point of water.
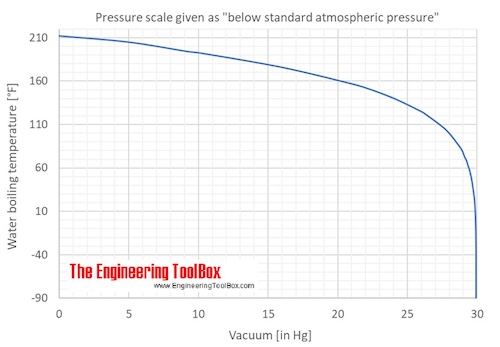
Water Boiling Points At Vacuum Pressure
The boiling point of a liquid varies depending upon the surrounding environmental pressure.
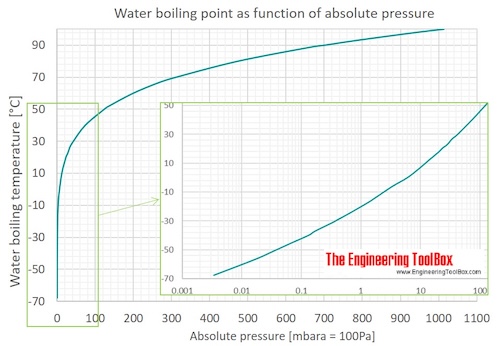
. NH3 SbH3 AsH3 PH3. A liquid in a partial vacuum has a lower boiling point than. The normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure 760 mm 2992 inches of mercury.
The normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure 760 mm 2992 inches of mercury. The boiling point of a liquid depends on temperature atmospheric pressure and the vapor pressure of the liquid. The boiling point of a liquid varies depending upon the surrounding environmental pressure.
A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. At sea level water boils at 100 C 212 F. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure.
At sea level water boils at 100 C 212 F. The boiling point of a liquid varies depending upon the surrounding environmental pressure. The pressure at which vaporization boiling starts to occur for a given temperature is called the saturation pressure.
The boiling point of water or any liquid varies according to the surrounding. For a given pressure different liquids will boil at different temperatures. The triple point for a substance varies with the pressure.
A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. The boiling point of a liquid varies depending upon the surrounding environmental pressure. In other words the boiling point of a liquid varies depending upon the surrounding environmental pressure.
The normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure 760 mm 2992 inches of mercury. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
The boiling point of a liquid varies with the surrounding atmospheric pressure. The normal boiling point of a compound is an indicator of the volatility of that compound. The boiling point of a liquid varies according to the applied pressure.
The boiling point of a liquid varies according to the applied pressure. The hydrides of group 5A are NH3 PH3 AsH3 and SbH3. Also know what is meant by the boiling point of a substance.
The boiling point of a liquid varies depending upon the surrounding environmental pressure. If pressure decreases boilin. View the full answer.
The temperature of a boiling liquid remains constant even when more heat is added. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies directly with the pressure above the liquid.
For a given pressure different liquids boil at different temperatures. The higher the boiling point the less volatile is the compound. The boiling point of a given liquid varies with pressure.
Does boiling water always have the same temperature. A liquid at high. Identify which of the following molecules can exhibit hydrogen bonding as a pure liquid.
The boiling point of a liquid varies depending upon the surrounding environmental pressure. Who are the experts. A liquid in a vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
A liquid in a vacuum has a lower boiling point than when that liquid is at atmospheric pressure. H3C-O-OH H2N-NH2 H3C-C O-O-H. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The greater the pressure the higher the boiling point. A liquid at high-pressure has a higher boiling point than when that liquid is at atmospheric pressure. The trend in boiling points is best attributed to.
Click to see full answer. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above itThe normal boiling point of a liquid is the temperature at which its vapor pressure is equal to one atmosphere 760 torr. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure.
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. For example water boils at 100 C 212 F at sea level but at 934 C 2001 F at 1905 metres 6250 ft altitude. The normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure 760 mm 2992 inches of mercury.
At higher altitudes the temperature of the boiling point is lower. At sea level water boils at 100 C. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
The boiling point of a given liquid varies. When the atmospheric pressure is equal to the vapor pressure of the liquid boiling will begin23 Sept 2020. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure.
The three phases solid liquid gas have the same Question. A liquid in a partial vacuum has a. When considered as the temperature of the reverse change from vapor to liquid it is called the condensation point.
The formulas and boiling points of three compounds are given in the table. Arrange them from highest to lowest boiling point. The boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at enviornment of liquid and the liquid changes to vapor.
A liquid at high. The boiling point of a liquid varies according to the applied pressure. We review their content and use your feedback to keep the quality high.
Experts are tested by Chegg as specialists in their subject area. A liquid at a higher pressure has a higher boiling point than when that liquid is at lower atmospheric pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
The boiling point of a liquid varies depending upon the surrounding environmental pressure. For example water boils at 100 C 212 F. The boiling point of a liquid varies according to the applied pressure.
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.

This Graph Shows How The Vapor Pressure Of Three Liquids Varies With Temperature Vapor Pressure Torr Homeworklib

Aleks Relating Vapor Pressure To Vaporization Youtube
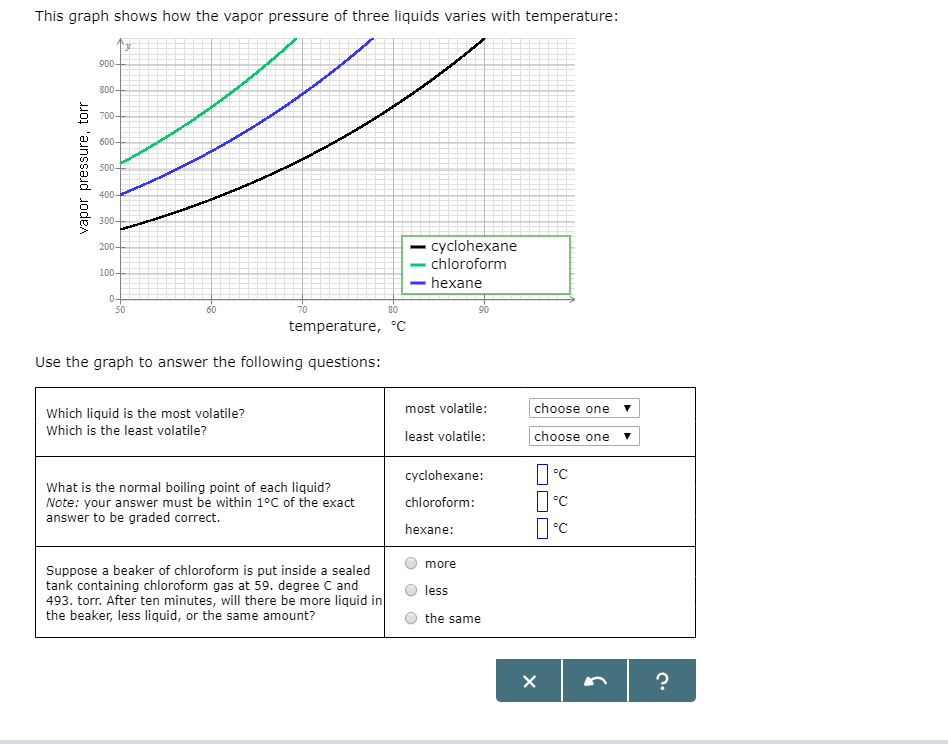
Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com
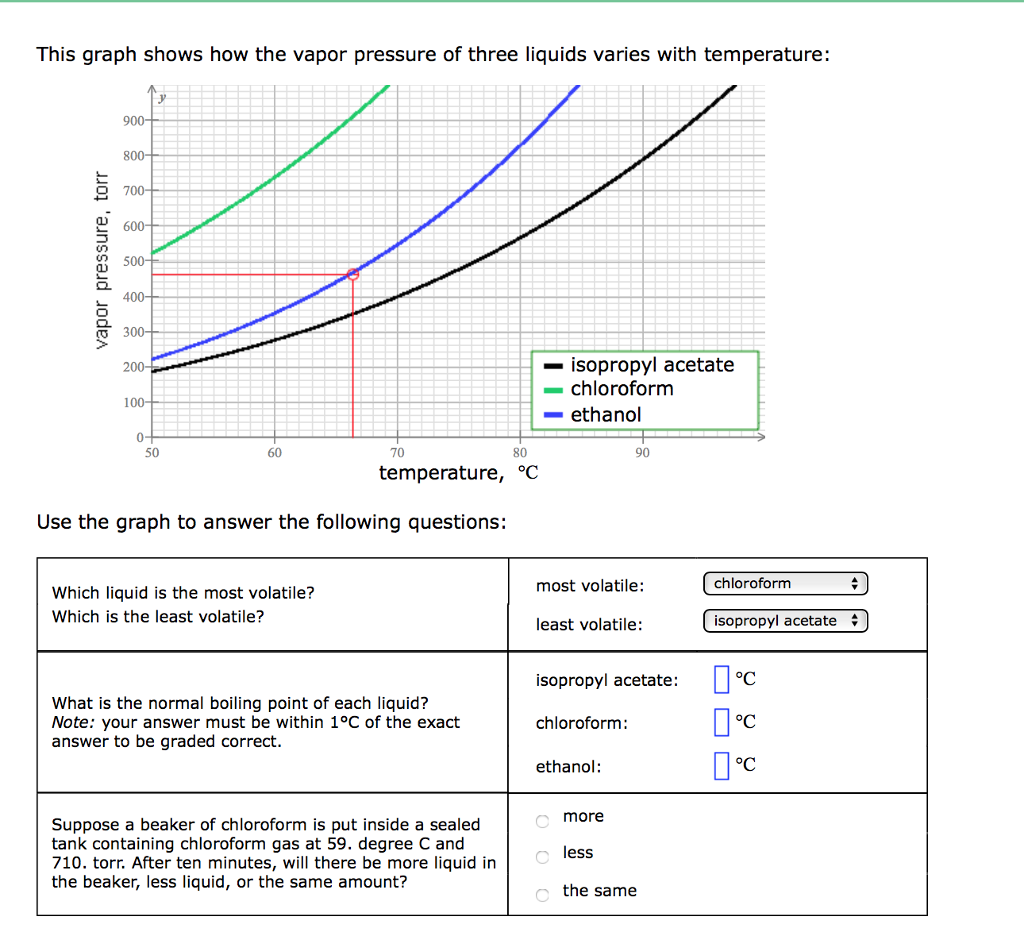
Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com
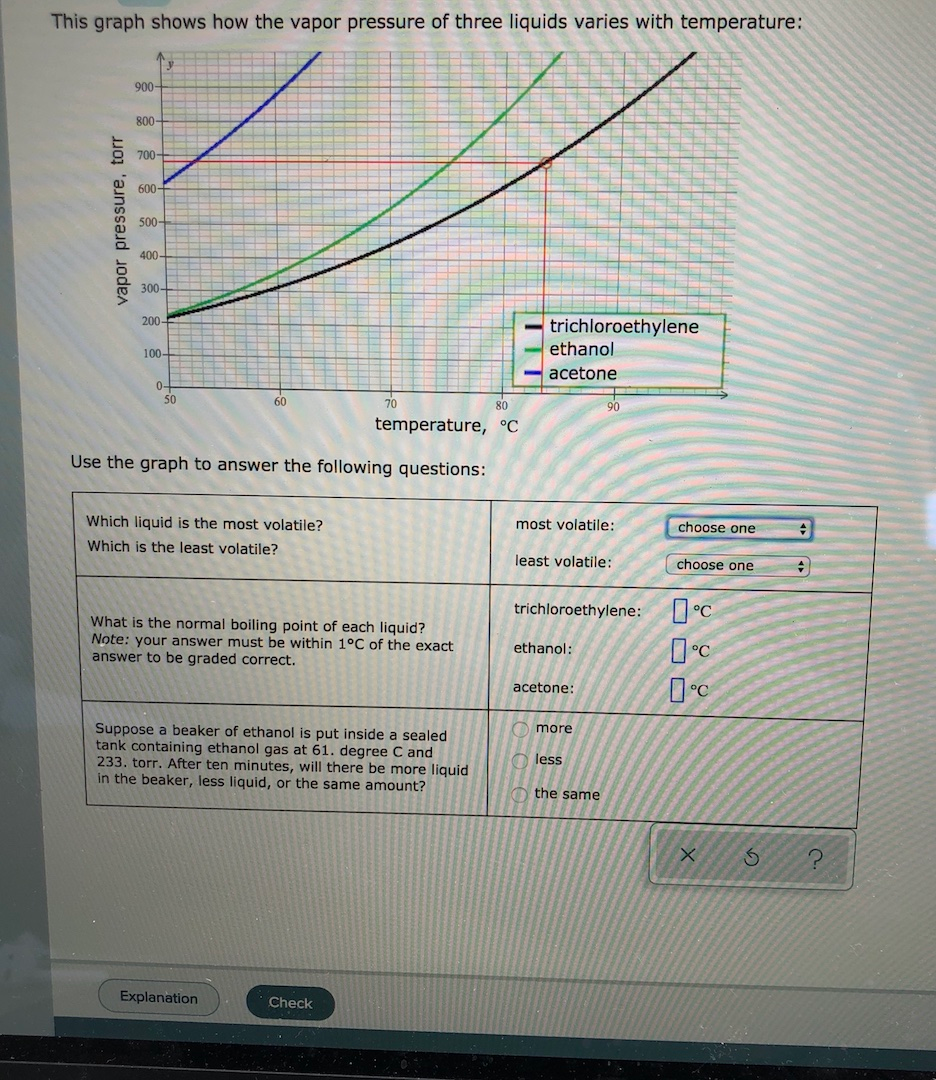
Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com

This Graph Shows How The Vapor Pressure Of Three Liquids Varies With Temperature Vapor Pressure Torr Homeworklib

Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com
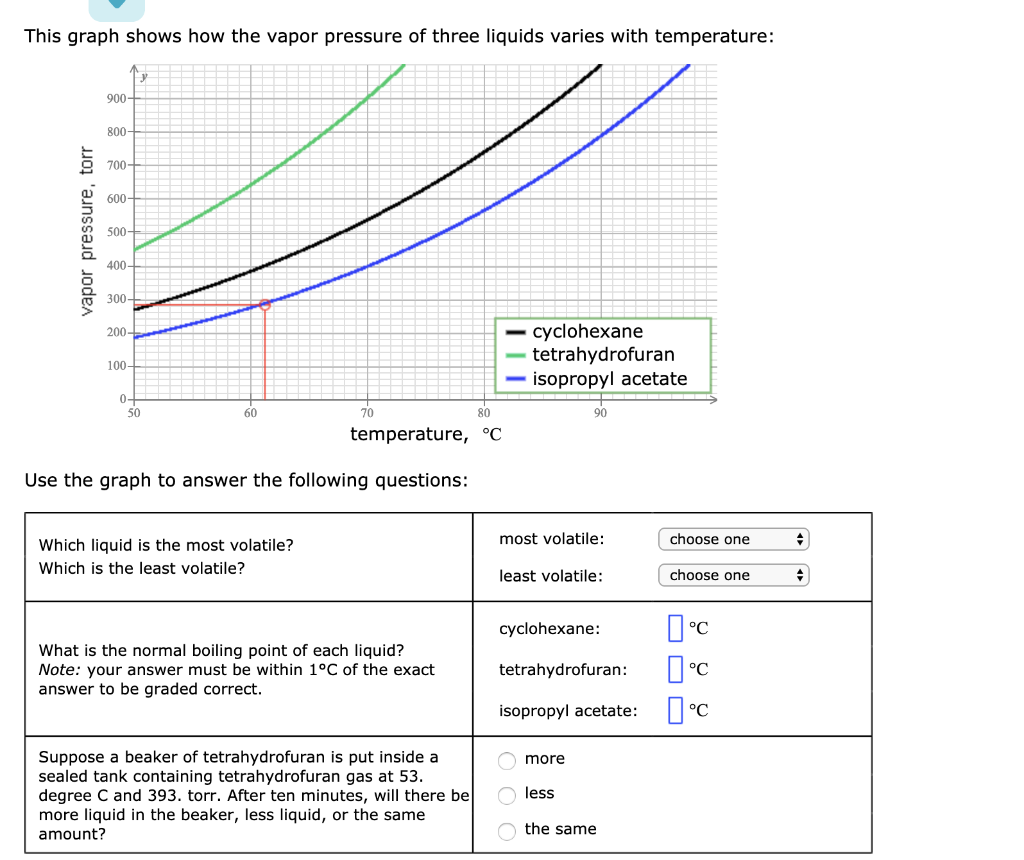
Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com

This Graph Shows How The Vapor Pressure Of Three Liquids Varies With Temperature Vapor Pressure Torr Homeworklib

This Graph Shows How The Vapor Pressure Of Three Liquids Varies With Temperature Vapor Pressure Torr Homeworklib

Learn About Boiling Point Of Water Chegg Com
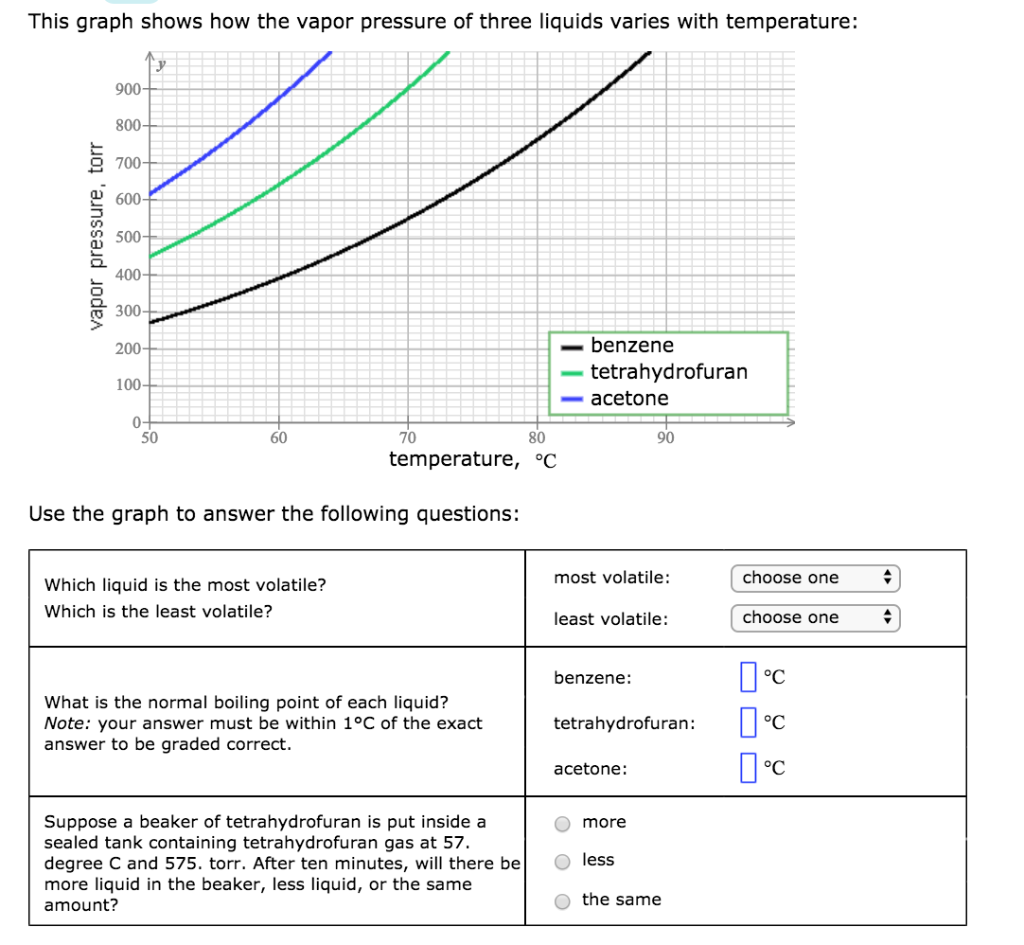
Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com

Water Boiling Points At Vacuum Pressure
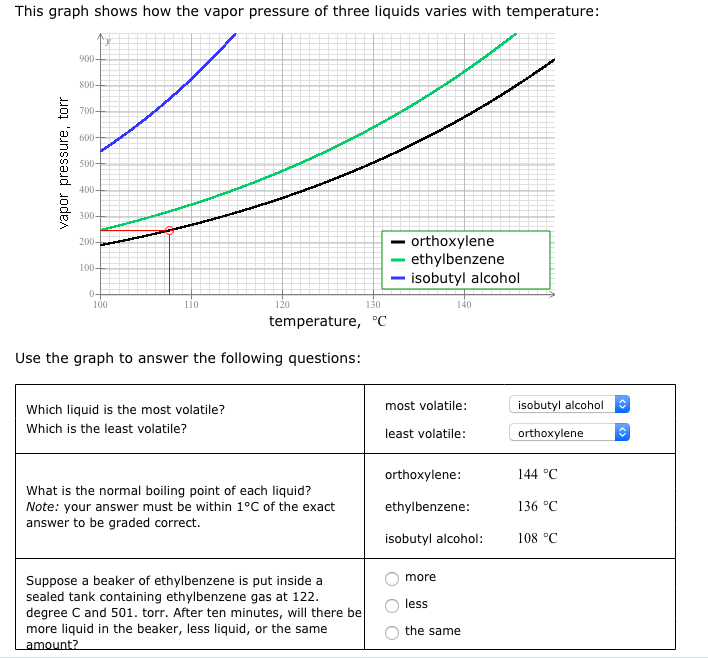
Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com

11 5 Vaporization And Vapor Pressure Chemistry Libretexts

Learn About Boiling Point Of Water Chegg Com

Solved This Graph Shows How The Vapor Pressure Of Three Chegg Com


Comments
Post a Comment